New type of plastic prevents plastic soup in ocean
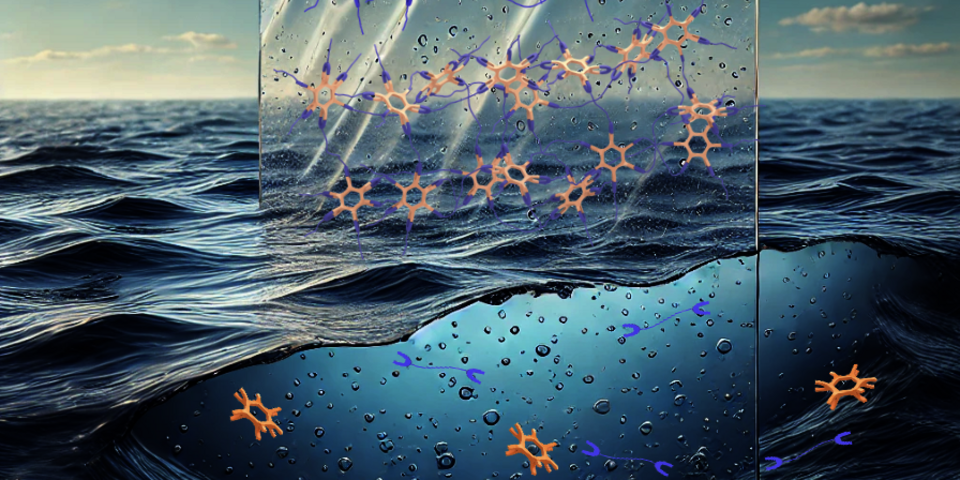
Professors Bert Meijer (TU/e) and Takuzo Aida (University of Tokyo) developed a new plastic that dissolves in salt water. They recently presented this in the journal Science. When this plastic with salt bridges comes into contact with salt water, the molecules fall apart again and are metabolized by microorganisms.
“Most of the work that went into this was done in Japan,” Meijer stresses. “But we’ve been working together for decades. What’s great is that Aida focuses more on the innovative research, the fireworks. I'm more concerned with the fundamental questions and how you can generalize things. So we complement each other very nicely.”
Meijer: “We can make plastic by mixing very ordinary simple salts in water and then we get a phase separation between an aqueous plastic layer and the water layer with the counter-ions of the building blocks. When the separation of the two layers is followed by a drying process, the molecules are connected by many ‘salt bridges’ and you end up with the plastic. It’s very hard and therefore very suitable for all kinds of applications for which plastic with a lot of carbon is currently used.” It is hard and strong, properties for which current plastic is also praised. But with this plastic with salt bridges, the molecules fall apart again when they come into contact with salt water and are either metabolized by micro-organisms or ready to be recycled.
Bring it to market
This new invention is naturally receiving wide attention: from the BBC to the Dutch daily newspaper Telegraaf. It has huge potential, of course: if everyone were to start using this new type of plastic, we would no longer have plastic soup in the ocean. But it’s precisely that broad implementation that presents the biggest challenge. “The price is the greatest stumbling block. This plastic based on salt bridges is more expensive than polyethylene plastic, which is actually far too cheap. And our new plastic is just as hard and strong as the plastic we know.” The price will therefore have to be paid if we don’t want to pollute the sea even more.
Meijer: “Plastic is cheap and practical, making it a widely used material. But we cannot continue like this, because the pollution caused by plastic is also enormous. China, Africa, and India are currently growing very quickly. As a result, even more things are being made from cheap plastic and this will only get worse in the years ahead.
In the not too distant future, we’ll have three times as much plastic as we do now. We must prevent that.” But how? Meijer thinks that an external stimulus is needed. “For example, if the government makes non-biodegradable plastic more expensive, it becomes much less attractive to the industry and consumers. Or do it the other way around: subsidize plastics that can be recycled.”
Incidentally, the plastic produced by the research groups of Aida and Meijer hasn’t been integrated into products yet. “We have tested it extensively as a material, for example when it comes to adhesiveness,” says Meijer. He finds the adhesive function of plastic very interesting. “I am often asked whether ‘debonding on demand’ is possible. In other words, whether you can stop the sticking whenever you want. And that’s now possible when it comes into contact with salt. As long as salt is absent, the material is actually very stable. Aida holds the patent for this is in Tokyo.”
This new type of plastic can be produced on a large scale, according to Meijer. And in terms of sustainability, it has another advantage. “It contains a very low amount of carbon, much lower than in polyethylene plastics. That’s of course better for the climate.”
Applications
The material has been tested extensively, but Meijer hopes this news will inspire companies to come up with new ideas for product applications. “It would be great if someone were to think ‘this is exactly what I am looking for’. That happened, for example, with our research into supramolecular polymers. L’Oréal turned out to be interested in it for their lipstick. I am the last person who would have thought of our research results being integrated into a new lipstick.”
The professor is aware that it’s difficult to conduct highly fundamental materials research while simultaneously thinking about the application in a product. “Everyone has to do what they do best. When I was researching our supramolecular polymers, I never thought it would become a biomaterial and now it’s actually in people (as part of artificial valves developed by TU/e spin-off Xeltis, ed.). That is the strength of the university: coming up with new things and inspiring people to pick those up and convert them into applications.”

Discussion