Home Stretch | Cooling red-hot steel with warm water
Cooling red-hot steel, after it has been flat-rolled into slabs of the desired thickness, is a rather delicate operation. PhD candidate Camila Gomez copied the cooling process of Tata Steel’s blast furnaces in her lab, and learned that using warmer water can be better.
In the blast furnaces at Tata Steel in IJmuiden, thick slabs of steel with a temperature of around twelve hundred degrees Celsius are flat-rolled, in various stages, from slabs with a thickness of approximately 20 centimeters to just a few centimeters. Within a few seconds these slabs have to be cooled to such an extent that they can be rolled into something resembling a huge toilet paper roll.
In order to cool the steel, the slabs pass underneath some sort of waterfalls at high speed, Camila Gomez explains. “The water that falls on the steel starts to boil and rapidly extracts heat from the material. The exact speed and uniformity with which the slab cools, will determine the material’s eventual characteristics, that is why it’s a rather delicate process.”
High speed
During the rolling process, the steel slabs grow in length from twenty to more than two hundred meters, which is also why the speed at which they move increases tenfold. “At the end, the steel passes through the jets of water at a speed of almost eighty kilometers per hour; since the cooling process thus takes place at an extremely high speed, it’s difficult to study it at the factory.”
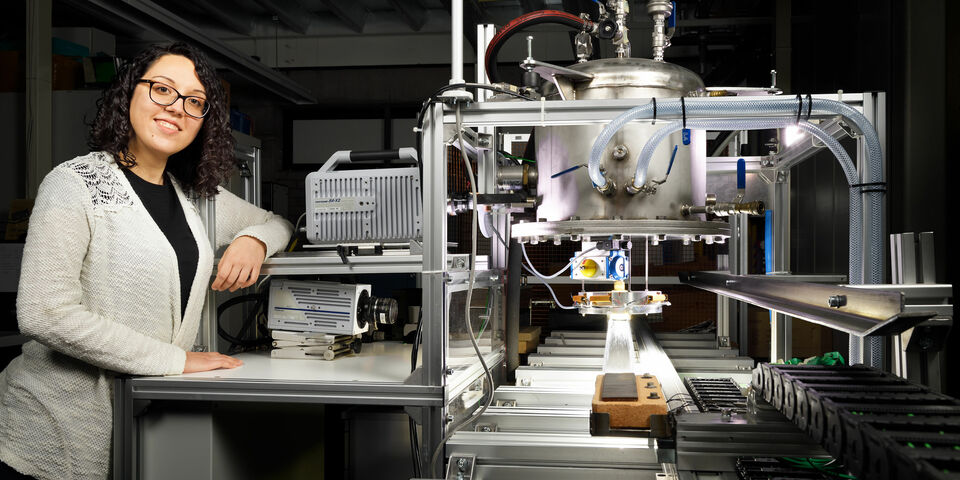
Still, in order to better adjust the industrial process to the production of new types of steel, you want to know exactly what happens to the cooling water near the steel’s surface. That is why Gomez - who was born in Argentina and emigrated in Spain at the age of ten - was asked, within a collaboration between the NWO, Tata Steel and TU/e, to make a test setup that makes it possible to analyze in detail how the cooling water starts to boil once it comes into contact with hot steel.
Text continues below the video.
“Until now, there had been only experiments using stationary and slow-moving setups,” Gomez says. “We’ve now built a setup in the lab in Gemini that allows us to move a piece of hot steel underneath a jet of water at a speed of almost thirty kilometers per hour as we make recordings close to the surface using high-speed cameras.” She used a so-called borescope for this, comparable to an endoscope for internal medical examination of the body, which she placed in the jets of water.
It had been established, the PhD candidate says, that the cooling water can come into contact with steel with a temperature of no less than nine hundred degrees. “That was a kind of mystery, because you would expect the water to heat up rapidly to three hundred degrees, after which it evaporates in an explosive manner. We’ve now seen that vapor bubble formation does indeed occur locally, but that these bubbles subsequently implode because the cold water falls down on them. That occurs up to forty thousand times per second - a process you can detect only when you record at a high frame rate and study those images one by one.”
That discovery is the greatest scientific result of her doctoral project, as far as Gomez is concerned. However, on top of that there is another discovery that might have serious practical implications. When the surface does not cool uniformly due to constant local vapor explosions, this will result in imperfections and steel of lesser quality. “Therefore, you want to cool the steel as evenly as possible. Our measurements show that warmer cooling water allows you to create a stable water vapor layer above the steel. Admittedly, that slows down the cooling process, but it does produce a better result.”
When Gomez raised the water temperature from twenty-five to sixty degrees, she was able in her test setup to cool the steel a further fifty degrees without entering the unstable regime. Knowledge that could be of high value to steel manufacturers, she says, since the water temperature can be easily adjusted without having to alter the entire production line.
Very special day
Her unique setup will surely be used by the next generation of students and PhD candidates, Gomez says. She herself will look for a job in industry. But not before she enters into marriage, on the very same day of her PhD defense ceremony, with her Miguel, with whom she recently bought a house in Eindhoven. Her family will travel to the Netherlands from Spain that day, if the corona measures allow it. “We hope that they’ll be present both at the PhD defense ceremony and our marriage.”



Discussion